Technical
With the support of our European collaborative organization, we maintain an ingredient specific protocol library for bio burden reduction of numerous dried food types used in multiple industries with diverse requirements. This allows us to maintain the stability of volatile oils, natural moisture content, and true color, assuring you of a superior process producing the safest and highest quality products for your manufacturing needs.
- Product is Taken to Lab for Microbial Load Testing to Determine Product Specific Protocols.
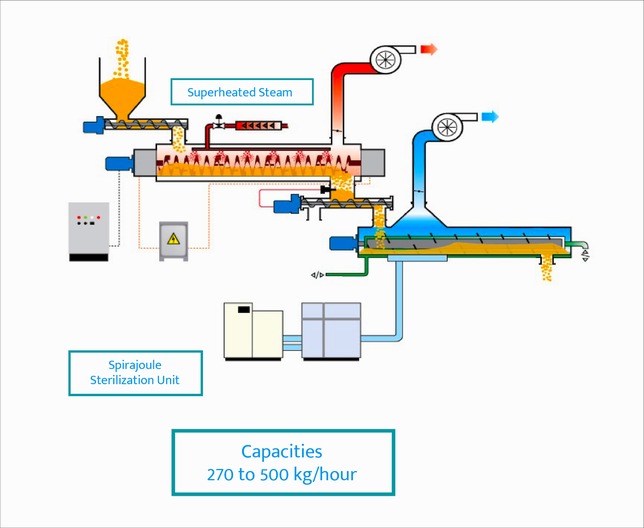
- Product is Loaded into Hopper
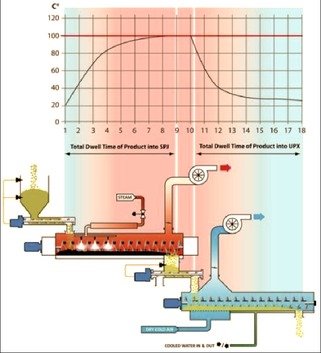
- Using Continuous Dosing Feed Mechanism Product is Fed to a Patented Closed Chamber that is Housed with a Heated Moving Auger Screw (between 90 – 300 ° C)
- While Product is Moved by Screw in the Chamber, Super Heated Dry ( or wet ) Steam is Injected into Chamber
- Combination of Dry Screw Heat and Dry / Wet Steam Reduces the Microbial Load
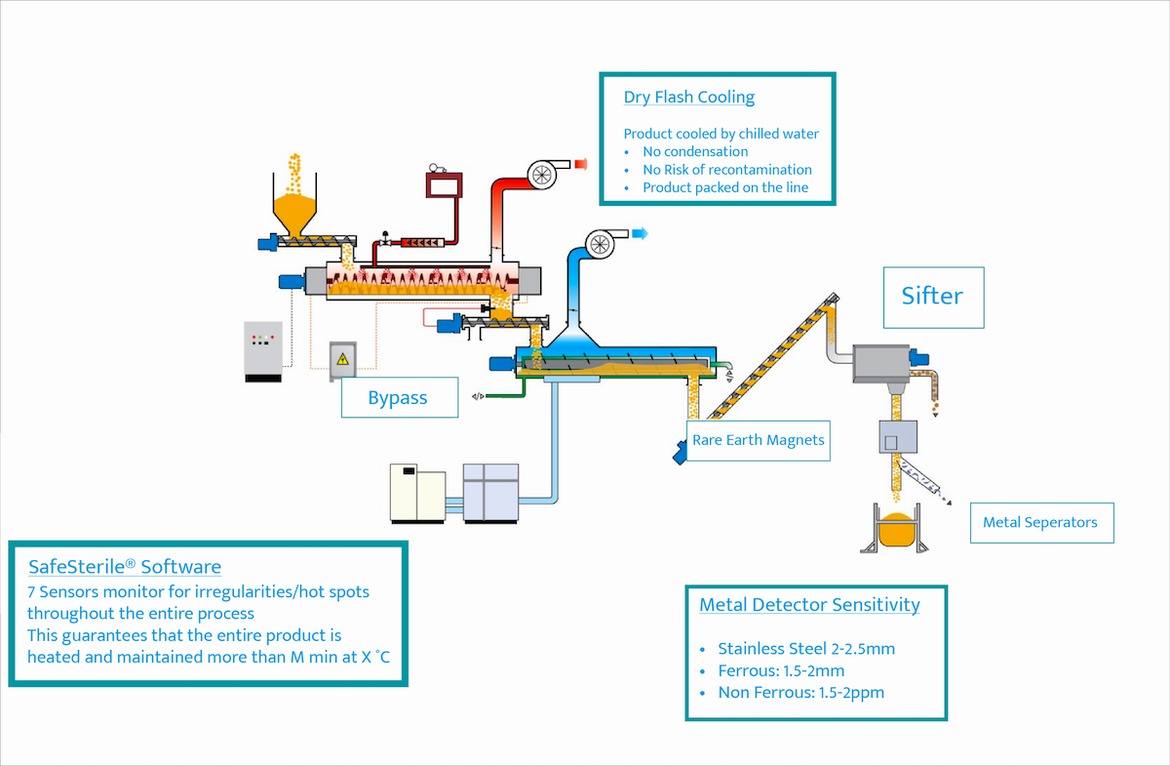
- At the Other End Treated Product is Loaded in to a Patented Cooling Chamber for Flash Cooling. This Flash Cooling will Further Reduce Microbial Load and Complete Validated Kill Step Requirements
- All Products Are Subject to Sifting and Metal Detection to Further Ensure Safety at the End of the Cooling Process
- Product is then Collected at the End of Cooling Unit and Packed
Through this control, it is possible to monitor and do traceability of the heat treatment, while respecting the products’ sensitivity and the decontamination target.
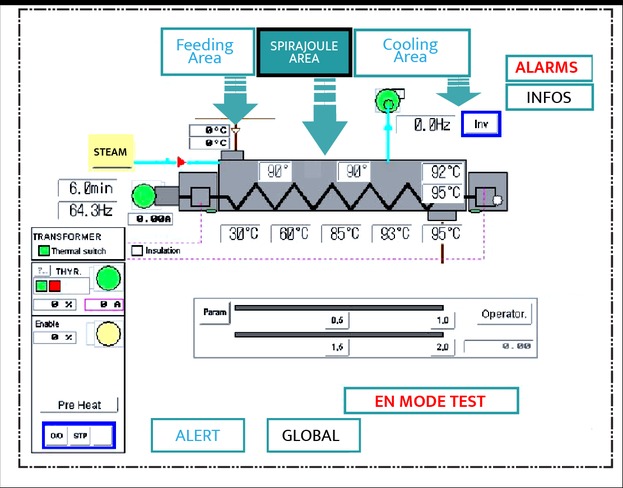
Operating parameters are recorded
Critical Control Points (CCP) follow-up
Guarantee that the entire product is heated & maintained more than M min at X °C
Benefits of the Continuous Process
- Saturated Steam
- Super Heated Dry Steam
- No Steam at All
- Can be used for Various Heat Treatments:
- Pasteurization
- Sterilization
- Roasting, Drying, Blanching
No restriction as per product shape
Capacities : 270 to 400 kg/h
Value Added Services At No Additional Cost
- Repackaging options from 15kg to 500kg
- Magnetic Sifting
- Full Compliment of Safety Certifications
- Gluten Free /Low Allergen Facility in Nevada
- Laboratory Services
- Microbiological Analysis
- Pathogen Analysis
Performance:
Chamomile Volatile Oils & Moisture

Chamomile Volatile Oils & Moisture
| Volatile Oil | Moisture | |
|---|---|---|
| Before Treatment | 0.37% | 10.8% |
| After Treatment | 0.35% | 10.3% |
If you’d like more info, you can download our info packet here
SSUSA Info Packet 2023
PDF – 4.1 MB 533 downloads
Before And After Images of Processed Ingredients


















Advantages
- Traditional ingredient harvesting creates opportunities for microbial pathogen exposure.
- Ingredient contamination levels can be as high as 10 Billion parts per gram
- Iodizing radiation may compromise color & flavor of ingredients with unclear long term health I’mpacts,
- The lagging of safety regulations being implemented on ingredient sterilization in the process food sector.
- The need for the protection of ingredient integrity, which are so sensitive to heat and moisture.
- The industry needed a better more advanced solution for treating organics.
- A continuous process -vs- the dated batch process.
- Integrity of both flavor & taste with less than 8% of oil content loss
- Color remains intact with minimal change in vibrancy
- No increase in moisture during the process ( even though it is a water based system!)
- The ability to treat a wide variety of product shapes, forms and sizes.
- The ability to complete 5-6 tons per cycle ( per location).
- Highly sensitive rare earth magnets and sifting at the end of the process as additional safety precautions.
- Two strategically located facilities with ability to service product coming through ports and hubs on both coasts.
- Stringent quality and safety controls in place.
- Third party validation by Novolyze.
- HACCP practices and standards maintained.
- Validation by Certified Labs.
- A full facility dedicated to low allergen/gluten free processing.
